Molecular Cell | The Shi Yigong team of Westlake University has made new progress! Analyze the splicing mechanism of human...
Date:2023-09-02
Scientists have long known that mitochondria play an important role in the metabolism and energy production of cancer cells. However, as of now, researchers are not clear about the relationship between the structural organization of mitochondrial networks and their functional bioenergy activity at the entire tumor level.
Recently, in a research report entitled "Spatial mapping of mitochondrial networks and bioenergetics in lung cancer" published on the international journal Nature, scientists from UCLA and other institutions combined Positron emission tomography (PET) and electron microscope through research, A three-dimensional super resolution map of mitochondrial network was generated in the Lung tumor of genetically engineered mice.
In the article, researchers used an artificial intelligence technique called deep learning to classify and analyze tumors based on mitochondrial activity and other factors, and quantified the structure of hundreds of cells and thousands of mitochondria throughout the entire tumor. Researchers studied two major subtypes of non-small cell lung cancer (NSCLC) - Adenocarcinoma of the lung and squamous cell carcinoma, and found different mitochondrial network subsets in these tumors; More importantly, they also found that mitochondria can often be organized together with Organelle such as lipid droplets and produce special subcellular structures, while supporting tumor cell metabolism and mitochondrial activity.
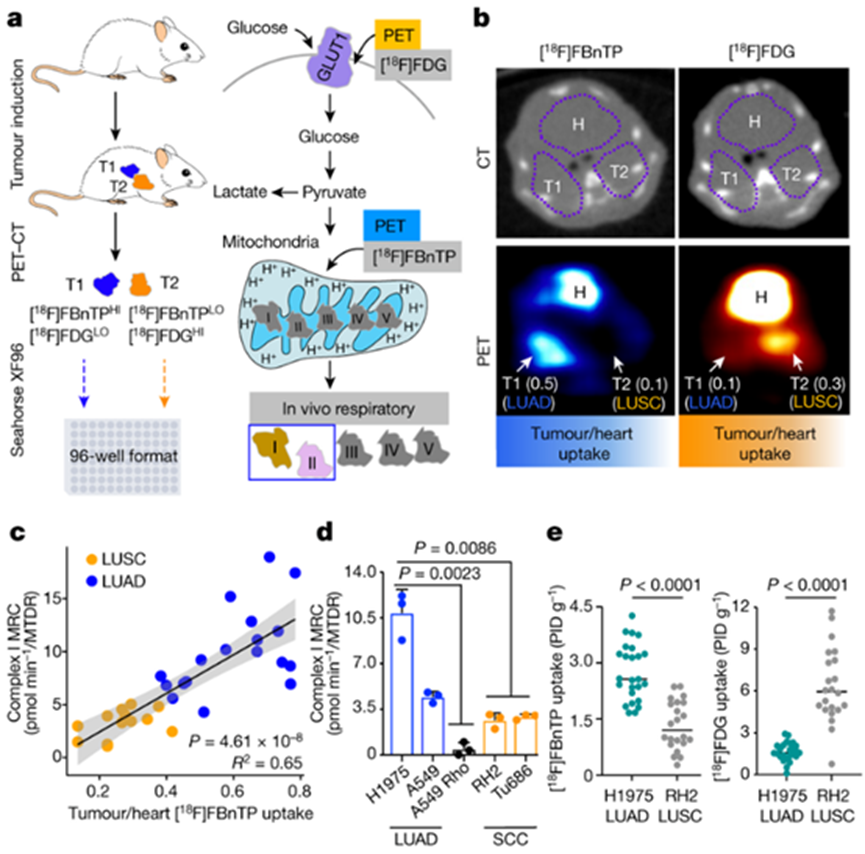 Image source: Nature (2023) DOI: 10.1038/s41586-023-05793-3
Image source: Nature (2023) DOI: 10.1038/s41586-023-05793-3
Researchers speculate that the mitochondrial population in human cancer samples does not repel their respective tumor subtypes, but rather has an activity spectrum; These research findings may provide key information for understanding the function of mitochondria in cancer cells and have the potential to help develop new cancer therapies. Shackelford, the researcher, said that our research represents the key first step to generate a highly detailed three-dimensional map of Lung tumor using a genetically engineered mouse model; Using these maps, we can generate a blueprint of the structure and function of Lung tumor, and provide valuable clues to reveal how tumor cells structurally organize their cellular architecture to respond to the high metabolic demands of tumor growth. Our research findings may help guide and improve current cancer treatment strategies, and also clarify the new direction of scientists' treatment of lung cancer.
This study reveals a new discovery of metabolic flux of Lung tumor, and clarifies that the preference of cancer cells for nutrition may be determined by the regions of mitochondria and other Organelle in their cells, that is, they either depend on glucose or free fatty acids. The research results of this article are of great significance for developing effective anticancer therapies that can target tumor specific nutritional preferences. This multimodal imaging method can also prompt researchers to uncover previously unknown aspects of cancer metabolism. Researchers believe that this may also be applied to research on other types of cancer.
In summary, the results of this study indicate that in non-small cell lung cancer, mitochondrial networks can be divided into different subpopulations and dominate the tumor's bioenergy capacity.